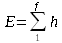
2014 05 05
Mike Polioudakis
How to use Planck’s constant
This note is a complaint I have against notation in physics. The notation leads to a mistaken mindset.
h = Planck’s constant
f = wave frequency
w = wave length (usually “l”)
f = 1/w
Ordinarily physicists write for energy of a wave:
E = f * h
This is fine as long as we remember that f is an integer and that h is discrete. “h” is not a real number in the ordinary sense of real numbers of being potentially infinitesimally small as part of a continuum. If h could be expressed as an integer, it should be expressed that way.
In its simple form, the common equation allows us to think of fractional frequencies and so to avoid the “making discrete” that is the point of Planck’s constant. Suppose all water came only in full pint glasses, so you could only get water in multiples of whole pints. The total amount of water would be:
Total water = pint unit * total pints ordered
“pint unit” is like “Planck’s constant” while “total pints ordered” is like frequency, with frequency specified as whole amounts (integers) of frequency.
People get around the making discrete by using fractional pints. In that way, they have any small amount of water, that is, water is effectively continuous. I think physicists often think of the energy formula in this way, forget they should not use fractional frequencies, and make energy continuous again.
When I see the common form of the energy equation, I am reminded of the equations for hyperbolas that I learned in high school, where all the numbers were real numbers, and could vary continuously. That is not the case.
It might be better, but more clumsy, to write the energy rule as
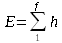
Energy = SUM, from 1 to f, of h discrete units of energy (or work or action)
With sigma notation, we can’t have fractional frequencies and continuous amounts of energy.
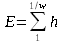
Instead of E = h*f, I prefer E = h * 1/w because, hopefully, waves can come only in whole units, and we are forced not to use fractional units, that is, fractional wavelengths.
Also, this way we can see that, as the wave length gets shorter, the energy density increases.
This attitude is not likely to catch on, but it is worth knowing.